Seed

A seed (/ˈsiːd/) is a small embryonic plant enclosed in a covering called the seed coat, usually with some stored food. It is the product of the ripened ovule of gymnosperm and angiosperm plants which occurs after fertilization and some growth within the mother plant. The formation of the seed completes the process of reproduction in seed plants (started with the development of flowers and pollination), with the embryo developed from the zygote and the seed coat from the integuments of the ovule.
Seeds have been an important development in the reproduction and spread of flowering plants, relative to more primitive plants like mosses, ferns and liverworts, which do not have seeds and use other means to propagate themselves. This can be seen by the success of seed plants (both gymnosperms and angiosperms) in dominating biological niches on land, from forests to grasslands both in hot and cold climates.
The term seed also has a general meaning that predates the above — anything that can be sown i.e. "seed" potatoes, "seeds" of corn or sunflower "seeds". In the case of sunflower and corn "seeds", what is sown is the seed enclosed in a shell or hull, and the potato is a tuber.
Contents |
Seed structure
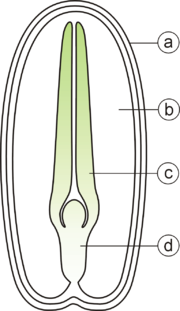
A typical seed includes three basic parts: (1) an embryo, (2) a supply of nutrients for the embryo, and (3) a seed coat.
The embryo is an immature plant from which a new plant will grow under proper conditions. The embryo has one cotyledon or seed leaf in monocotyledons, two cotyledons in almost all dicotyledons and two or more in gymnosperms. The radicle is the embryonic root. The plumule is the embryonic shoot. The embryonic stem above the point of attachment of the cotyledon(s) is the epicotyl. The embryonic stem below the point of attachment is the hypocotyl.
Within the seed, there usually is a store of nutrients for the seedling that will grow from the embryo. The form of the stored nutrition varies depending on the kind of plant. In angiosperms, the stored food begins as a tissue called the endosperm, which is derived from the parent plant via double fertilization. The usually triploid endosperm is rich in oil or starch and protein. In gymnosperms, such as conifers, the food storage tissue is part of the female gametophyte, a haploid tissue. In some species, the embryo is embedded in the endosperm or female gametophyte, which the seedling will use upon germination. In others, the endosperm is absorbed by the embryo as the latter grows within the developing seed, and the cotyledons of the embryo become filled with this stored food. At maturity, seeds of these species have no endosperm and are termed exalbuminous seeds. Some exalbuminous seeds are bean, pea, oak, walnut, squash, sunflower, and radish. Seeds with an endosperm at maturity are termed albuminous seeds. Most monocots (e.g. grasses and palms) and many dicots (e.g. brazil nut and castor bean) have albuminous seeds. All gymnosperm seeds are albuminous.
The seed coat (or testa) develops from the tissue, the integument, originally surrounding the ovule. The seed coat in the mature seed can be a paper-thin layer (e.g. peanut) or something more substantial (e.g. thick and hard in honey locust and coconut). The seed coat helps protect the embryo from mechanical injury and from drying out.
In addition to the three basic seed parts, some seeds have an appendage on the seed coat such an aril (as in yew and nutmeg) or an elaiosome (as in Corydalis) or hairs (as in cotton). There may also be a scar on the seed coat, called the hilum; it is where the seed was attached to the ovary wall by the funiculus.
Seed production

Seeds are produced in several related groups of plants, and their manner of production distinguishes the angiosperms ("enclosed seeds") from the gymnosperms ("naked seeds"). Angiosperm seeds are produced in a hard or fleshy structure called a fruit that encloses the seeds, hence the name. (Some fruits have layers of both hard and fleshy material). In gymnosperms, no special structure develops to enclose the seeds, which begin their development "naked" on the bracts of cones. However, the seeds do become covered by the cone scales as they develop in some species of conifer.
Seed production in natural plant populations vary widely from year-to-year in response to weather variables, insects and diseases, and internal cycles within the plants themselves. Over a 20-year period, for example, forests composed of loblolly pine and shortleaf pine produced from 0 to nearly 5 million sound pine seeds per hectare.[1] Over this period, there were six bumper seeds, five poor seeds crops, and nine good seed crops, when evaluated in regard to producing adequate seedlings for natural forest reproduction.
Kinds of seeds
Many structures commonly referred to as "seeds" are actually dry fruits. Sunflower seeds are sold commercially while still enclosed within the hard wall of the fruit, which must be split open to reach the seed. Different groups of plants have other modifications, the so-called stone fruits (such as the peach) have a hardened fruit layer ( the endocarp) fused to and surrounding the actual seed. Nuts are the one-seeded, hard shelled fruit, of some plants, with an indehiscent seed, such as an acorn or hazelnut.
Seed development

The seed, which is an embryo with two points of growth (one of which forms the stems the other the roots) is enclosed in a seed coat with some food reserves. Angiosperm seeds consist of three genetically distinct constituents: (1) the embryo formed from the zygote, (2) the endosperm, which is normally triploid, (3) the seed coat from tissue derived from the maternal tissue of the ovule. In angiosperms, the process of seed development begins with double fertilization and involves the fusion of the egg and sperm nuclei into a zygote. The second part of this process is the fusion of the polar nuclei with a second sperm cell nucleus, thus forming a primary endosperm. Right after fertilization, the zygote is mostly inactive but the primary endosperm divides rapidly to form the endosperm tissue. This tissue becomes the food that the young plant will consume until the roots have developed after germination or it develops into a hard seed coat. The seed coat forms from the two integuments or outer layers of cells of the ovule, which derive from tissue from the mother plant, the inner integument forms the tegmen and the outer forms the testa. When the seed coat forms from only one layer it is also called the testa, though not all such testa are homologous from one species to the next.
In gymnosperms, the two sperm cells transferred from the pollen do not develop seed by double fertilization but one sperm nucleus unites with the egg nucleus and the other sperm is not used.[2] Sometimes each sperm fertilizes an egg cell and one zygote is then aborted or absorbed during early development.[3] The seed is composed of the embryo (the result of fertilization) and tissue from the mother plant, which also form a cone around the seed in coniferous plants like Pine and Spruce.
The ovules after fertilization develop into the seeds; the main parts of the ovule are the funicle; which attaches the ovule to the placenta, the nucellus; the main region of the ovule were the embryo sac develops, the micropyle; A small pore or opening in the ovule where the pollen tube usually enters during the process of fertilization, and the chalaza; the base of the ovule opposite the micropyle, where integument and nucellus are joined together.[4]
The shape of the ovules as they develop often affects the finale shape of the seeds. Plants generally produce ovules of four shapes: the most common shape is called anatropous, with a curved shape. Orthotropous ovules are straight with all the parts of the ovule lined up in a long row producing an uncurved seed. Campylotropous ovules have a curved embryo sac often giving the seed a tight “c” shape. The last ovule shape is called amphitropous, where the ovule is partly inverted and turned back 90 degrees on its stalk or funicle.
In the majority of flowering plants, the zygote's first division is transversely oriented in regards to the long axis, and this establishes the polarity of the embryo. The upper or chalazal pole becomes the main area of growth of the embryo, while the lower or micropylar pole produces the stalk-like suspensor that attaches to the micropyle. The suspensor absorbs and manufacturers nutrients from the endosperm that are utilized during the embryos growth.[5]
The embryo is composed of different parts; the epicotyle will grow into the shoot, the radicle grows into the primary root, the hypocotyl connects the epicotyle and the radicle, the cotyledons form the seed leaves, the testa or seed coat forms the outer covering of the seed. Monocotyledonous plants like corn, have other structures; instead of the hypocotyle-epicotyle, it has a coleoptile that forms the first leaf and connects to the coleorhiza that connects to the primary root and adventitious roots form from the sides. The seeds of corn are constructed with these structures; pericarp, scutellum (single large cotyledon) that absorbs nutrients from the endosperm, endosperm, plumule, radicle, coleoptile and coleorhiza - these last two structures are sheath-like and enclose the plumule and radicle, acting as a protective covering. The testa or seed coats of both monocots and dicots are often marked with patterns and textured markings, or have wings or tufts of hair.
Seed size and seed set
Seeds are very diverse in size. The dust-like orchid seeds are the smallest with about one million seeds per gram; they are often embryonic seeds with immature embryos and no significant energy reserves. Orchids and a few other groups of plants are myco-heterotrophs which depend on mycorrhizal fungi for nutrition during germination and the early growth of the seedling. Some terrestrial Orchid seedlings, in fact, spend the first few years of their life deriving energy from the fungus and do not produce green leaves.[6] At over 20 kg, the largest seed is the coco de mer. Plants that produce smaller seeds can generate many more seeds per flower, while plants with larger seeds invest more resources into those seeds and normally produce fewer seeds. Small seeds are quicker to ripen and can be dispersed sooner, so fall blooming plants often have small seeds. Many annual plants produce great quantities of smaller seeds; this helps to ensure that at least a few will end in a favorable place for growth. Herbaceous perennials and woody plants often have larger seeds, they can produce seeds over many years, and larger seeds have more energy reserves for germination and seedling growth and produce larger, more established seedlings after germination.[7][8]
Seed functions
Seeds serve several functions for the plants that produce them. Key among these functions are nourishment of the embryo, dispersal to a new location, and dormancy during unfavorable conditions. Seeds fundamentally are a means of reproduction and most seeds are the product of sexual reproduction which produces a remixing of genetic material and phenotype variability that natural selection acts on.
Embryo nourishment
Seeds protect and nourish the embryo or young plant. Seeds usually give a seedling a faster start than a sporeling from a spore, because of the larger food reserves in the seed and the multicellularity of the enclosed embryo.
Seed dispersal
Unlike animals, plants are limited in their ability to seek out favorable conditions for life and growth. As a result, plants have evolved many ways to disperse their offspring by dispersing their seeds (see also vegetative reproduction). A seed must somehow "arrive" at a location and be there at a time favorable for germination and growth. When the fruits open and release their seeds in a regular way, it is called dehiscent, which is often distinctive for related groups of plants, these fruits include; Capsules, follicles, legumes, silicles and siliques. When fruits do not open and release their seeds in a regular fashion they are called indehiscent, which include the fruits achenes, caryopsis, nuts, samaras, and utricles.[9]
Seed dispersal is seen most obviously in fruits; however many seeds aid in their own dispersal. Some kinds of seeds are dispersed while still inside a fruit or cone, which later opens or disintegrates to release the seeds. Other seeds are expelled or released from the fruit prior to dispersal. For example, milkweeds produce a fruit type, known as a follicle,[10] that splits open along one side to release the seeds. Iris capsules split into three "valves" to release their seeds.[11]
By wind (anemochory)


- Many seeds (e.g. maple, pine) have a wing that aids in wind dispersal.
- The dustlike seeds of orchids are carried efficiently by the wind.
- Some seeds, (e.g. dandelion, milkweed, poplar) have hairs that aid in wind dispersal.[12]
Some winged seeds have two, and some have only one wing.
By water (hydrochory)
- Some plants, such as Mucuna and Dioclea, produce buoyant seeds termed sea-beans or drift seeds because they float in rivers to the oceans and wash up on beaches.[13]
By animals (zoochory)
- Seeds (burrs) with barbs or hooks (e.g. acaena, burdock, dock) which attach to animal fur or feathers, and then drop off later.
- Seeds with a fleshy covering (e.g. apple, cherry, juniper) are eaten by animals (birds, mammals, reptiles, fish) which then disperse these seeds in their droppings.
- Seeds (nuts) which are an attractive long-term storable food resource for animals (e.g. acorns, hazelnut, walnut); the seeds are stored some distance from the parent plant, and some escape being eaten if the animal forgets them.
Myrmecochory is the dispersal of seeds by ants. Foraging ants disperse seeds which have appendages called elaiosomes[14] (e.g. bloodroot, trilliums, Acacias, and many species of Proteaceae). Elaiosomes are soft, fleshy structures that contain nutrients for animals that eat them. The ants carry such seeds back to their nest, where the elaiosomes are eaten. The remainder of the seed, which is hard and inedible to the ants, then germinates either within the nest or at a removal site where the seed has been discarded by the ants.[15] This dispersal relationship is an example of mutualism, since the plants depend upon the ants to disperse seeds, while the ants depend upon the plants seeds for food. As a result, a drop in numbers of one partner can reduce success of the other. In South Africa, the Argentine ant (Linepithema humile) has invaded and displaced native species of ants. Unlike the native ant species, Argentine ants do not collect the seeds of Mimetes cucullatus or eat the elaiosomes. In areas where these ants have invaded, the numbers of Mimetes seedlings have dropped.[16]
Seed dormancy
Seed dormancy has two main functions: the first is synchronizing germination with the optimal conditions for survival of the resulting seedling; the second is spreading germination of a batch of seeds over time so that a catastrophe after germination (e.g. late frosts, drought, herbivory) does not result in the death of all offspring of a plant (bet-hedging).[17] Seed dormancy is defined as a seed failing to germinate under environmental conditions optimal for germination, normally when the environment is at a suitable temperature with proper soil moisture. This true dormancy or innate dormancy is therefore caused by conditions within the seed that prevent germination. Thus dormancy is a state of the seed, not of the environment.[18] Induced dormancy, enforced dormancy or seed quiescence occurs when a seed fails to germinate because the external environmental conditions are inappropriate for germination, mostly in response to conditions being too dark or light, too cold or hot, or too dry.
Seed dormancy is not the same as seed persistence in the soil or on the plant, though even in scientific publications dormancy and persistence are often confused or used as synonyms.[19]
Often seed dormancy is divided into four major categories: exogenous; endogenous; combinational; and secondary. A more recent system distinguishes five classes of dormancy:morphological, physiological, morphophysiological, physical and combinational dormancy.[20]
Exogenous dormancy is caused by conditions outside the embryo including:
- Physical dormancy or hard seed coats occurs when seeds are impermeable to water. At dormancy break a specialized structure, the ‘water gap’, is disrupted in response to environmental cues, especially temperature, so that water can enter the seed and germination can occur. Plant families where physical dormancy occurs include Anacardiaceae, Cannaceae, Convulvulaceae, Fabaceae and Malvaceae.[21]
- Chemical dormancy considers species that lack physiological dormancy, but where a chemical prevents germination. This chemical can be leached out of the seed by rainwater or snow melt or be deactivated somehow.[22] Leaching of chemical inhibitors from the seed by rain water is often cited as an important cause of dormancy release in seeds of desert plants, however little evidence exists to support this claim.[23]
Endogenous dormancy is caused by conditions within the embryo itself, including:
- Morphological dormancy where germination is prevented due to morphological characteristics of the embryo. In some species the embryo is just a mass of cells when seeds are dispersed, it is not differentiated. Before germination can take place both differentiation and growth of the embryo have to occur. In other species the embryo is differentiated but not fully grown (underdeveloped) at dispersal and embryo growth up to a species specific length is required before germination can occur. Examples of plant families where morphological dormancy occurs are Apiaceae, Cycadaceae, Liliaceae, Magnoliaceae and Ranunculaceae.[24][25]
- Morphophysiological dormancy seeds with underdeveloped embryos, and which in addition have physiological components to dormancy. These seeds therefore require a dormancy-breaking treatments as well as a period of time to develop fully grown embryos. Plant families where morphophysiological dormancy occurs include Apiaceae, Aquifoliaceae, Liliaceae, Magnoliaceae, Papaveraceae and Ranunculaceae.[24] Some plants with morphophysiological dormancy like Asarum or Trillium species have multiple types of dormancy, one affects radicle (root) growth while the other affects plumule (shoot) growth. The terms "double dormancy" and "2-year seeds" are used for species whose seeds need two years to complete germination or at least two winters and one summer. Dormancy of the radicle (seedling root)is broken during the first winter after dispersal while dormancy of the shoot bud is broken during the second winter.[24]
- Physiological dormancy means that the embryo can, due to physiological causes, not generate enough power to break through the seed coat, endosperm or other covering structures. Dormancy is typically broken at cool wet, warm wet or warm dry conditions. Abscisic acid is usually the growth inhibitor in seeds and its production can be affected by light.
- Drying; some plants including a number of grasses and those from seasonally arid regions need a period of drying before they will germinate, the seeds are released but need to have a lower moisture content before germination can begin. If the seeds remain moist after dispersal, germination can be delayed for many months or even years. Many herbaceous plants from temperate climate zones have physiological dormancy that disappears with drying of the seeds. Other species will germinate after dispersal only under very narrow temperature ranges, but as the seeds dry they are able to germinate over a wider temperature range.[26]
- Combinational dormancy In seeds with combinational dormancy the seed or fruit coat is impermeable to water and the embryo has physiological dormancy. Depending on the species physical dormancy can be broken before or after physiological dormancy is broken.[25]
- Secondary dormancy* is caused by conditions after the seed has been dispersed and occurs in some seeds when non-dormant seed is exposed to conditions that are not favorable to germination, very often high temperatures. The mechanisms of secondary dormancy are not yet fully understood but might involve the loss of sensitivity in receptors in the plasma membrane.[27]
The following types of seed dormancy do not involve seed dormancy strictly spoken as lack of germination is prevented by the environment not by characteristics of the seed itself (see Germination):
- Photodormancy or light sensitivity affects germination of some seeds. These photoblastic seeds need a period of darkness or light to germinate. In species with thin seed coats, light may be able to penetrate into the dormant embryo. The presence of light or the absence of light may trigger the germination process, inhibiting germination in some seeds buried too deeply or in others not buried in the soil.
- Thermodormancy is seed sensitivity to heat or cold. Some seeds including cocklebur and amaranth germinate only at high temperatures (30C or 86F) many plants that have seed that germinate in early to mid summer have thermodormancy and germinate only when the soil temperature is warm. Other seeds need cool soils to germinate, while others like celery are inhibited when soil temperatures are too warm. Often thermodormancy requirements disappear as the seed ages or dries.
Not all seeds undergo a period of dormancy. Seeds of some mangroves are viviparous, they begin to germinate while still attached to the parent. The large, heavy root allows the seed to penetrate into the ground when it falls. Many garden plants have seeds that will germinate readily as soon as they have water and are warm enough, though their wild ancestors may have had dormancy, these cultivated plants lack seed dormancy. After many generations of selective pressure by plant breeders and gardeners dormancy has been selected out.
For annuals, seeds are a way for the species to survive dry or cold seasons. Ephemeral plants are usually annuals that can go from seed to seed in as few as six weeks.[28]
Seed persistence and seed banks
Seed germination

Seed germination is a process by which a seed embryo develops into a seedling. It involves the reactivation of the metabolic pathways that lead to growth and the emergence of the radicle or seed root and plumule or shoot. The emergence of the seedling above the soil surface is the next phase of the plants growth and is called seedling establishment.[29]
Three fundamental conditions must exist before germination can occur. (1) The embryo must be alive, called seed viability. (2) Any dormancy requirements that prevent germination must be overcome. (3) The proper environmental conditions must exist for germination.
Seed viability is the ability of the embryo to germinate and is affected by a number of different conditions. Some plants do not produce seeds that have functional complete embryos or the seed may have no embryo at all, often called empty seeds. Predators and pathogens can damage or kill the seed while it is still in the fruit or after it is dispersed. Environmental conditions like flooding or heat can kill the seed before or during germination. The age of the seed affects its health and germination ability: since the seed has a living embryo, over time cells die and cannot be replaced. Some seeds can live for a long time before germination, while others can only survive for a short period after dispersal before they die.
Seed vigor is a measure of the quality of seed, and involves the viability of the seed, the germination percentage, germination rate and the strength of the seedlings produced.[30]
The germination percentage is simply the proportion of seeds that germinate from all seeds subject to the right conditions for growth. The germination rate is the length of time it takes for the seeds to germinate. Germination percentages and rates are affected by seed viability, dormancy and environmental effects that impact on the seed and seedling. In agriculture and horticulture quality seeds have high viability, measured by germination percentage plus the rate of germination. This is given as a percent of germination over a certain amount of time, 90% germination in 20 days, for example. 'Dormancy' is covered above; many plants produce seeds with varying degrees of dormancy, and different seeds from the same fruit can have different degrees of dormancy.[31] It's possible to have seeds with no dormancy if they are dispersed right away and do not dry (if the seeds dry they go into physiological dormancy). There is great variation amongst plants and a dormant seed is still a viable seed even though the germination rate might be very low.
Environmental conditions effecting seed germination include; water, oxygen, temperature and light.
Three distinct phases of seed germination occur: water imbibition; lag phase; and radicle emergence.
In order for the seed coat to split, the embryo must imbibe (soak up water), which causes it to swell, splitting the seed coat. However, the nature of the seed coat determines how rapidly water can penetrate and subsequently initiate germination. The rate of imbibition is dependent on the permeability of the seed coat, amount of water in the environment and the area of contact the seed has to the source of water. For some seeds, imbibing too much water too quickly can kill the seed. For some seeds, once water is imbibed the germination process cannot be stopped, and drying then becomes fatal. Other seeds can imbibe and lose water a few times without causing ill effects, but drying can cause secondary dormancy.
Inducing germination
A number of different strategies are used by gardeners and horticulturists to break seed dormancy.
Scarification which allows water and gases to penetrate into the seed, include methods that physically break the hard seed coats or soften them by chemicals. Means of scarification include soaking in hot water or poking holes in the seed with a pin or rubbing them on sandpaper or cracking with a press or hammer. Soaking the seeds in solvents or acids is also effective for many seeds. Sometimes fruits are harvested while the seeds are still immature and the seed coat is not fully developed and sown right away before the seed coat become impermeable. Under natural conditions seed coats are worn down by rodents chewing on the seed, the seeds rubbing against rocks (seeds are moved by the wind or water currents), by undergoing freezing and thawing of surface water, or passing through an animal's digestive tract. In the latter case, the seed coat protects the seed from digestion, while often weakening the seed coat such that the embryo is ready to sprout when it gets deposited (along with a bit of fertilizer) far from the parent plant. Microorganisms are often effective in breaking down hard seed coats and are sometimes used by people as a treatment, the seeds are stored in a moist warm sandy medium for several months under non-sterile conditions.
Stratification also called moist-chilling is a method to break down physiological dormancy and involves the addition of moisture to the seeds so they imbibe water and then the seeds are subject to a period of moist chilling to after-ripen the embryo. Sowing outside in late summer and fall and allowing to overwinter outside under cool conditions is an effective way to stratify seeds, some seeds respond more favorably to periods of osculating temperatures which are part of the natural environment.
Leaching or the soaking in water removes chemical inhibitors in some seeds that prevent germination. Rain and melting snow naturally accomplish this task. For seeds planted in gardens, running water is best - if soaked in a container, 12 to 24 hours of soaking is sufficient. Soaking longer, especially in stagnant water that is not changed, can result in oxygen starvation and seed death. Seeds with hard seed coats can be soaked in hot water to break open the impermeable cell layers that prevent water intake.
Other methods used to assist in the germination of seeds that have dormancy include prechilling, predrying, daily alternation of temperature, light exposure, potassium nitrate, the use of plant growth regulators like gibberellins, cytokinins, ethylene, thiourea, sodium hypochlorite plus others.[32] Some seeds germinate best after a fire, for some seeds fire cracks hard seed coats while in other seeds chemical dormancy is broken in reaction to the presence of smoke, liquid smoke is often used by gardeners to assist in the germination of these species.[33]
Origin and evolution
The origin of seed plants is a problem that still remains unsolved. However, more and more data tends to place this origin in the middle Devonian. The description in 2004 of the proto-seed Runcaria heinzelinii in the Givetian of Belgium is an indication of that ancient origin of seed-plants. As with modern ferns, most land plants before this time reproduced by sending spores into the air, that would land and become whole new plants.
The first "true" seeds are described from the upper Devonian, which is probably the theater of their true first evolutionary radiation. The seed plants progressively became one of the major elements of nearly all ecosystems.
Economic importance

Edible seeds
Many seeds are edible and the majority of human calories comes from seeds , especially from cereals, legumes and nuts. Seeds also provide most cooking oils, many beverages and spices and some important food additives. In different seeds the seed embryo or the endosperm dominates and provides most of the nutrients. The storage proteins of the embryo and endosperm differ in their amino acid content and physical properties. For example the gluten of wheat, important in providing the elastic property to bread dough is strictly an endosperm protein.
Seeds are used to propagate many crops such as cereals, legumes, forest trees, turfgrasses and pasture grasses. Particularly in developing countries, a major constraint faced is the inadequacy of the marketing channels to get the seed to poor farmers.[34] Thus the use of farmer-retained seed remains quite common.
Seeds are also eaten by animals, and are fed to livestock. Many seeds are used as birdseed.
Poison and food safety
While some seeds are edible, others are harmful, poisonous or deadly.[35] Plants and seeds often contain chemical compounds to discourage herbivores and seed predators. In some cases, these compounds simply taste bad (such as in mustard), but other compounds are toxic or break down into toxic compounds within the digestive system. Children, being smaller than adults, are more susceptible to poisoning by plants and seeds.[36]
A deadly poison, ricin, comes from seeds of the castor bean. Reported lethal doses are anywhere from two to eight seeds,[37][38] though only a few deaths have been reported when castor beans have been ingested by animals.[39]
In addition, seeds containing amygdalin—apple, apricot, bitter almond,[40] peach, plum, cherry, quince, and others—when consumed in sufficient amounts, may cause Cyanide poisoning.[40][41] Other seeds that contain poisons include annona, cotton, custard apple, datura, uncooked durian, golden chain, horse-chestnut, larkspur, locoweed, lychee, nectarine, rambutan, rosary pea, sour sop, sugar apple, wisteria, and yew.[42][43] The seeds of the strychnine tree are also poisonous, containing the poison strychnine.
The seeds of many legumes, including the common bean (Phaseolus vulgaris), contain proteins called lectins which can cause gastric distress if the beans are eaten without cooking. The common bean and many others, including the soybean, also contain trypsin inhibitors which interfere with the action of the digestive enzyme trypsin. Normal cooking processes degrade lectins and trypsin inhibitors to harmless forms.[44]
Please see the category plant toxins for further relevant articles.
Other uses

Cotton fiber grows attached to cotton plant seeds. Other seed fibers are from kapok and milkweed.
Many important nonfood oils are extracted from seeds. Linseed oil is used in paints. Oil from jojoba and crambe are similar to whale oil.
Seeds are the source of some medicines including castor oil, tea tree oil and the discredited cancer drug, Laetrile.
Many seeds have been used as beads in necklaces and rosaries including Job's tears, Chinaberry, rosary pea, and castor bean. However, the latter three are also poisonous.
Other seed uses include:
- Seeds once used as weights for balances.
- Seeds used as toys by children, such as for the game conker.
- Resin from Clusia rosea seeds used to caulk boats.
- Nematicide from milkweed seeds.
- Cottonseed meal used as animal feed and fertilizer.
Seed records

- The oldest viable carbon-14-dated seed that has grown into a plant was a Judean date palm seed about 2,000 years old, recovered from excavations at Herod the Great's palace on Masada in Israel. It was germinated in 2005.[45]
- The largest seed is produced by the coco de mer, or "double coconut palm", Lodoicea maldivica. The entire fruit may weigh up to 23 kilograms (50 pounds) and usually contains a single seed.[46]
- The earliest fossil seeds are around 365 million years old from the Late Devonian of West Virginia. The seeds are preserved immature ovules of the plant Elkinsia polymorpha.[47]
See also
- Brownbagging
- Biological dispersal
- Germination
- List of edible seeds
- Recalcitrant seed
- Seed company
- Seed enhancement
- Seed orchard
- Seed paper
- Seed predation
- Seed saving
- Seed testing
- Seedbed
- Seedling
- Soil seed bank
- Stratification
References
- ↑ Cain, M.D.; Shelton, M.G. 2001. Twenty years of natural loblolly and shortleaf pine seed production on the Crossett Experimental Forest in southeastern Arkansas. Southern Journal of Applied Forestry. 25(1): 40-45.
- ↑ Rost, Thomas L.; Weier, T. Elliot; Weier, Thomas Elliot (1979). Botany: a brief introduction to plant biology. New York: Wiley. pp. 319. ISBN 0-471-02114-8.
- ↑ Filonova LH, Bozhkov PV, von Arnold S (February 2000). "Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking". J Exp Bot. 51 (343): 249–64. doi:10.1093/jexbot/51.343.249. PMID 10938831. http://jxb.oxfordjournals.org/cgi/content/full/51/343/249.
- ↑ Galili G, Kigel J (1995). "Chapter One". Seed development and germination. New York: M. Dekker. ISBN 0-8247-9229-7.
- ↑ Raven, Peter H., Ray Franklin Evert, and Helena Curtis. 1981. Biology of plants. New York, N.Y.: Worth Publishers. page 410.
- ↑ Smith, Welby R. 1993. Orchids of Minnesota. Minneapolis: University of Minnesota Press. Page 8.
- ↑ Igor Kosinki (2007). "Long-term variability in seed size and seedling establishment of Maianthemum bifolium". Plant Ecology 194 (2): 149–156. doi:10.1007/s11258-007-9281-1. http://www.springerlink.com/content/1027t86224633171/.
- ↑ Shannon DA, Isaac L, Brockman FE (February 1996). "Assessment of hedgerow species for seed size, stand establishment and seedling height". Agroforestry Systems 35 (1): 95–110. doi:10.1007/BF02345331. http://www.springerlink.com/content/n537375121383705/?p=6bf47ea68f7f4a8fba3999fcf9da716e&pi=6.
- ↑ Jones, Samuel B., and Arlene E. Luchsinger. 1979. Plant systematics. McGraw-Hill series in organismic biology. New York: McGraw-Hill. Page 195.
- ↑ Cronquist, Arthur (1981). An Integrated System of Classification of Flowering Plants. New York: Columbia University Press. pp. 882. ISBN 0-231-03880-1.
- ↑ Stern, Kingsley R. (1991). Introductory Plant Biology (5th ed.). Dubuque, IA: Wm. C. Brown Publishers. pp. 131. ISBN 0-697-09947-4.
- ↑ Morhardt, Sia; Morhardt, Emil; Emil Morhardt, J. (2004). California desert flowers: an introduction to families, genera, and species. Berkeley: University of California Press. pp. 24. ISBN 0-520-24003-0. http://books.google.com/?id=1XyN-u-Bk40C&pg=PA24.
- ↑ www.seabean.com - Sea-Beans and Drift Seeds
- ↑ Marinelli, J. (1999) "Ants - The astonishing intimacy between ants & plants." Plants & Gardens News 14 (1). [1]
- ↑ Ricklefs, Robert E. (1993) The Economy of Nature, 3rd ed., p.396. (New York: W. H. Freeman). ISBN 0-7167-2409-X.
- ↑ Bond, W. J.; P. Slingsby (1984). "Collapse of an ant-plant mutualism: The Argentine ant, Iridomyrmex humilis and myrmecochorous Proteaceae". Ecology 65 (4): 1031–1037. doi:10.2307/1938311. http://jstor.org/stable/1938311.
- ↑ Eira MTS, Caldas LS (2000) Seed dormancy and germination as concurrent processes. Rev Bras Fisiol Vegetal 12:85–104
- ↑ Vleeshouwers, L.M., H.J. Bouwmeester & C.M. Karssen, 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83:1031-1037
- ↑ Thompson K, Ceriani RM, Bakker JP, Bekker RM (2003) Are seed dormancy and persistence in soil related? 13:97-100
- ↑ Baskin, J.M. and Baskin, C.C. (2004) A classification system for seed dormancy. Seed Science Research 14, 1–16
- ↑ Baskin JM, Baskin CC, Li X (2000) Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology 15:139-152
- ↑ Baskin, C.C. and Baskin, J.M. (1998) Seeds: Ecology, biogeography, and evolution of dormancy and germination.San Diego, Academic Press
- ↑ Gutterman, Y. (1993) Seed germination in desert plants. Springer Verlag, Berlin/Heidelberg.
- ↑ 24.0 24.1 24.2 Baskin, C.C. and Baskin, J.M. (1998) Seeds: Ecology, biogeography, and evolution of dormancy and germination.San Diego, Academic Press.
- ↑ 25.0 25.1 Baskin, J.M. and Baskin, C.C. (2004) A classification system for seed dormancy. Seed Science Research 14:1–16.
- ↑ International Workshop on Seeds, and G. Nicolas. 2003. The biology of seeds recent research advances : proceedings of the Seventh International Workshop on Seeds, Salamanca, Spain 2002. Wallingford, Oxon, UK: CABI Pub. Page 113.
- ↑ Bewley, J. Derek, and Michael Black. 1994. Seeds physiology of development and germination. The language of science. New York: Plenum Press. page 230.
- ↑ Patten, D.T. 1978. Productivity and production efficiency of an Upper Sonoran Desert ephemeral community. American Journal of Botany 65: 891-895.
- ↑ Black, Michael H.; Halmer, Peter (2006). The encyclopedia of seeds: science, technology and uses. Wallingford, UK: CABI. pp. 224. ISBN 978-0-85199-723-0.
- ↑ Seed Vigor and Vigor Tests
- ↑ International Seed Testing Association. 1973. ISSN 0251-0952. Pages 120-21.Seed science and technology. Wageningen?: International Seed Testing Association.
- ↑ Hartmann, Hudson Thomas, and Dale E. Kester. 1983. Plant propagation principles and practices. Englewood Cliffs, N.J.: Prentice-Hall. ISBN 0136810071. Pages 175-77.
- ↑ Trace Gas Emissions and Smoke-Induced Seed Germination - Keeley and Fotheringham 276 (5316): 1248 - Science
- ↑ G. Mumby Seed Marketing, FAO, Rome
- ↑ Chia Joo Suan, "Seeds of Doubt: Food Safety"
- ↑ Clelland, Mike. "Poisonous Plants and Seeds", Healthy Child Care
- ↑ Poisonous Plants
- ↑ Wedin GP, Neal JS, Everson GW, Krenzelok EP (May 1986). "Castor bean poisoning". Am J Emerg Med 4 (3): 259–61. doi:10.1016/0735-6757(86)90080-X. PMID 3964368.
- ↑ Albretsen JC, Gwaltney-Brant SM, Khan SA (2000). "Evaluation of castor bean toxicosis in dogs: 98 cases". J Am Anim Hosp Assoc 36 (3): 229–33. PMID 10825094. http://www.jaaha.org/cgi/pmidlookup?view=long&pmid=10825094.
- ↑ 40.0 40.1 Almond/Almond Oil
- ↑ Wolke, RL. Seeds of Anxiety Washington Post January 5, 2005
- ↑ Poisonous plants
- ↑ Chia Joo Suan Food Safety: Seeds of doubt
- ↑ Dhurandhar NV, Chang KC (1990). "Effect of Cooking on Firmness, Trypsin Inhibitors, Lectins and Cystine/Cysteine content of Navy and Red Kidney Beans (Phaseolus vulgaris)". J Food Sci. 55 (2): 470–4. doi:10.1111/j.1365-2621.1990.tb06789.x. http://www3.interscience.wiley.com/journal/119368153/abstract.
- ↑ Roach, John. (2005) "2,000-Year-Old Seed Sprouts, Sapling Is Thriving", National Geographic News, 22 November.
- ↑ Corner EJH (1966). The Natural History of Palms. Berkeley, CA: University of California Press. pp. 313–4.
- ↑ Taylor EL, Taylor TMC (1993). The biology and evolution of fossil plants. Englewood Cliffs, N.J: Prentice Hall. pp. 466. ISBN 0-13-651589-4.
External links
- List of Common Botanical Seed Names
- The Seed Site: collecting, storing, sowing, germinating, and exchanging seeds, with pictures of seeds, seedpods and seedlings.
- The Seed Biology Place seed structure, dormany, evolution, ecology, etc.
- Flavon's Secret Flower Garden - Pictures of Japanese plant seeds, fruits and etc.
- The Millennium Seed Bank Project Kew Garden's ambitious preservation project
- The Svalbard Global Seed Vault - a backup facility for the world's seed banks
|
|||||||||||||||||||||||||||||